
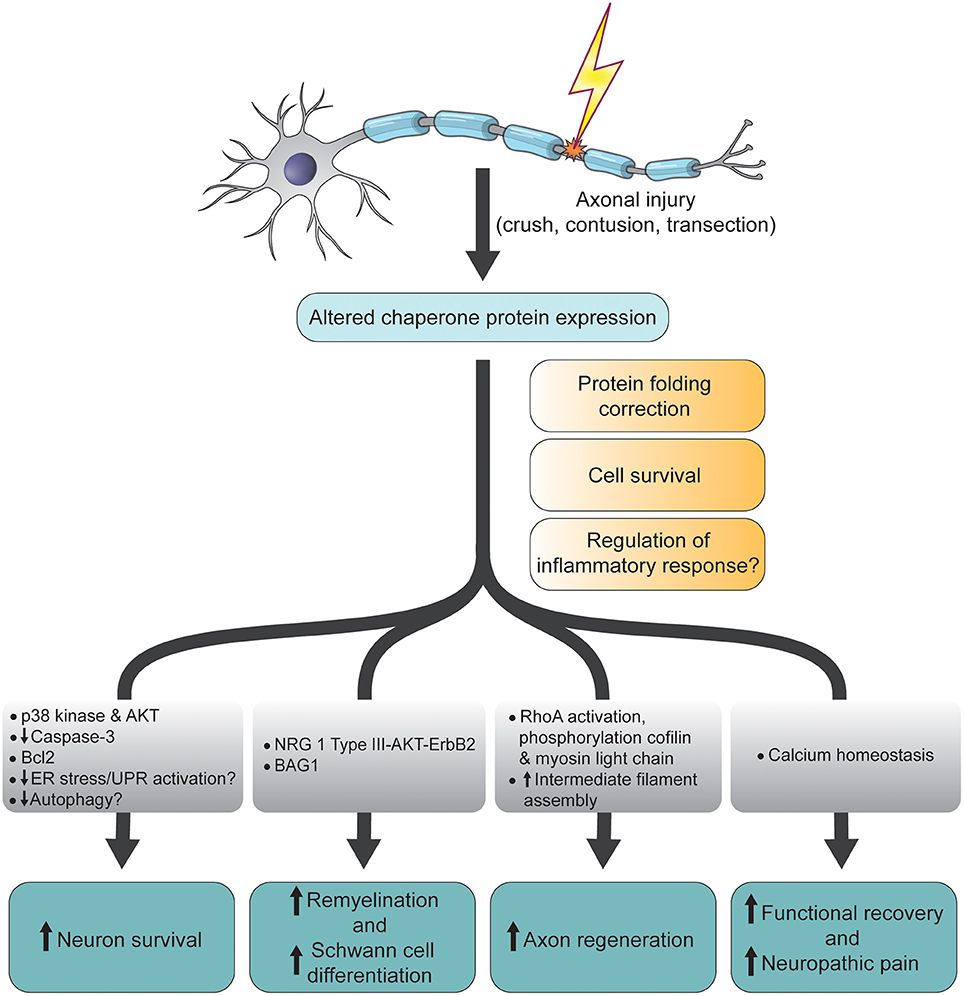
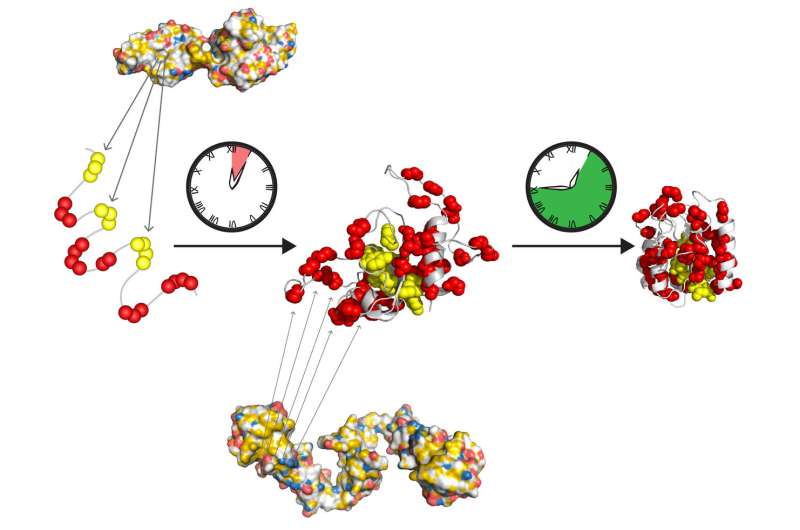
Each disorder is characterized by the misfolding of one or more specific proteins: amyloid-β (Aβ) and Tau (MAPT) in AD, α-synuclein (α-syn/SNCA) in PD, Huntingtin (HTT) in HD, superoxide dismutase 1 (SOD1), TAR DNA binding protein 43 (TDP-43/TARDBP), FUS RNA-binding protein (FUS) and dipeptide repeat proteins (DPRs) translated from C9orf72-SMCR8 complex subunit (C9orf72) in ALS, and the prion protein (PrP/PRNP) in prion diseases (Dobson, 2017 Eisenberg and Sawaya, 2017). Understanding how chaperones alleviate and aggravate disease progression is vital for the development of therapeutic strategies to combat these debilitating diseases.Ī common feature in many neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and prion diseases is the age-related formation of amyloid deposits (Chiti and Dobson, 2017). This review article focuses on the steps of prion-like propagation from initial misfolding and self-templated replication to intercellular spreading and discusses the influence that chaperones have on these various steps, highlighting both the positive and adverse consequences chaperone action can have. Recently, there has been a plethora of studies investigating how and when chaperones interact with disease-related proteins, which have advanced our understanding of the role of chaperones in protein misfolding diseases. With increasing age, however, the capacity of this proteostasis network tends to decrease, which enables the manifestation of neurodegenerative diseases. Molecular chaperones play a major role in maintaining cellular proteostasis by assisting the (re)-folding of cellular proteins to ensure their function or by promoting the degradation of terminally misfolded proteins to prevent damage. This has been attributed to a prion-like behavior of amyloid-type aggregates, which involves self-replication of the pathological conformation, intercellular transfer, and the subsequent seeding of native forms of the same protein in the neighboring cell. Pathological inclusions and the associated toxicity appear to spread through the nervous system in a characteristic pattern during the disease. Aberrant accumulation of misfolded proteins into amyloid deposits is a hallmark in many age-related neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS).


 0 kommentar(er)
0 kommentar(er)
